Co-reporter: Renee T. Williams and Yinsheng Wang
pp:
Publication Date(Web):July 18, 2012
DOI: 10.1021/bi300797q
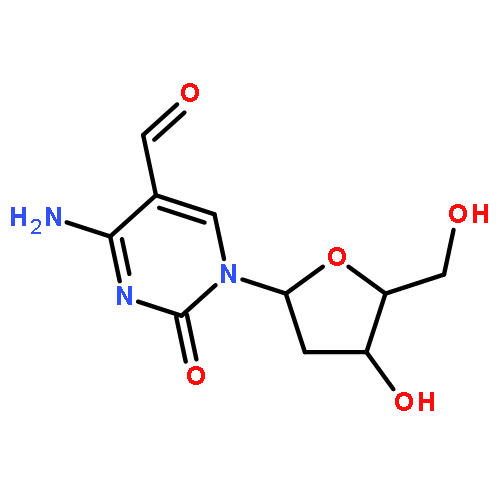
B3LYP/6-311+G(2d,p)//B3LYP/6-31+G(d) density functional theory calculations were employed to explore the kinetics and thermodynamics of gas-phase N-glycosidic bond cleavage induced by nucleophilic attack of C1′ with a hydroxide ion in 5-substituted 2′-deoxycytidines. The results showed that, among the 5-substituted 2′-deoxycytidine derivatives examined [XdC, where X = H (dC), CH3 (medC), CH2OH (hmdC), CHO (fmdC), COOH (cadC), F (FdC), or Br (BrdC)], fmdC and cadC exhibited the lowest energy barrier and largest exothermicity for N-glycosidic bond cleavage. These results paralleled previously reported nucleobase excision activities of human thymine DNA glycosylase (hTDG) toward duplex DNA substrates harboring a thymine and 5-substituted cytosine derivatives when paired with a guanine. Our study suggests that the inherent chemistry associated with the nucleophilic cleavage of N-glycosidic bond constitutes a major factor contributing to the selectivity of hTDG toward 5-substituted dC derivatives. These findings provided novel insights into the role of TDG in active cytosine demethylation.