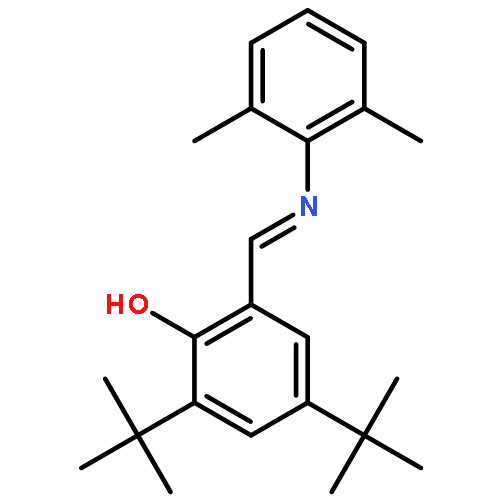
The synthesis and spectroscopy of divalent first row transition metals bearing two monoanionic salicylaldiminate ligands is reported. The reaction of MnCl2, FeCl2, CoBr2, 1,2-(Ph2PCH2CH2PPh2)NiCl2, and CuCl2 with 2 equiv. of the alkali metal salt of [OC6H2tBu2C(H)
![double bond; length as m-dash]()
N(C6H3Me2)]1− produces the corresponding M[OC6H2tBu2C(H)
![double bond; length as m-dash]()
N(C6H3Me2)]2, M = Mn, Fe, Co, Ni, and Cu. Reaction of ZnEt2 with 2 equiv. of the protonated ligand affords Zn[OC6H2tBu2C(H)
![double bond; length as m-dash]()
N(C6H3Me2)]2. The molecular structure of each complex has been analyzed using IR spectroscopy and X-ray crystallography while UV–Vis, CW-EPR, solution magnetic susceptibilities, and DFT calculations were also used to probe their electronic structure.The synthesis, structure, and spectroscopy of first row transition metal, Mn–Zn, complexes with two salicylaldiminate ligands are reported. The complexes exhibit non-covalent interactions which add stability to the tetrahedral geometry about the metal center instead of square planar which is observed if these interactions are absent.
![Image for unlabelled figure]()