Co-reporter: Anja S. Goldmann, Thomas Tischer, Leonie Barner, Michael Bruns, and Christopher Barner-Kowollik
pp:
Publication Date(Web):March 2, 2011
DOI: 10.1021/bm101461h
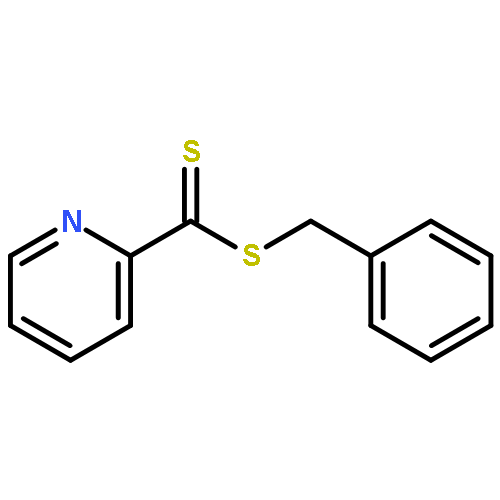
A combination of reversible addition−fragmentation chain transfer (RAFT) polymerization and hetero Diels−Alder (HDA) cycloaddition was used to effect, under mild (T ≈ 20 °C), fast, and modular conditions, the grafting of poly(isobornyl acrylate) (Mn = 9800 g mol−1, PDI = 1.19) onto a solid cellulose substrate. The active hydroxyl groups expressed on the cellulose fibers were converted to tosylate leaving groups, which were subsequently substituted by a highly reactive cyclopentadienyl functionality (Cp). By employing the reactive Cp-functionality as a diene, thiocarbonyl thio-capped poly(isobornyl acrylate) synthesized via RAFT polymerization (mediated by benzyl pyridine-2-yldithioformiate (BPDF)) was attached to the surface under ambient conditions by an HDA cycloaddition (reaction time: 15 h). The surface-modified cellulose samples were analyzed in-depth by X-ray photoelectron spectroscopy, scanning electron microscopy, elemental analysis, Fourier transform infrared (FT-IR) spectroscopy as well as Fourier transform infrared microscopy employing a focal plane array detector for imaging purposes. The analytical results provide strong evidence that the reaction of suitable dienophiles with Cp-functional cellulose proceeds under mild reaction conditions (T ≈ 20 °C) in an efficient fashion. In particular, the visualization of individual modified cellulose fibers via high-resolution FT-IR microscopy corroborates the homogeneous distribution of the polymer film on the cellulose fibers.